Ultrasonic instrument for optimal haemostasis in open liver surgery
The LOTUS liver resector for open surgery is specifically designed for use on liver parenchymal tissue. The large contact surface of the transducer ensures an increased haemostatic effect with reduced dissection speed.Liver resection for open surgery
With the ultrasonic scalpel, one blade of the instrument starts vibrating. With 36,000 torsional oscillations per second, not only is a dissection effect created, but also optimal haemostasis is achieved at the same time.BOWA MEDICAL LOTUS ultrasound instruments are ideal as a safe complement to or replacement for electrosurgery. It is used in general surgery, surgical gynaecology and urology, as well as thoracic surgery for sealing vessels up to 5 mm and for the precise dissection of tissue.
Product details:
- working length 176 mm
- straight jaws
- cross section 5.5 mm
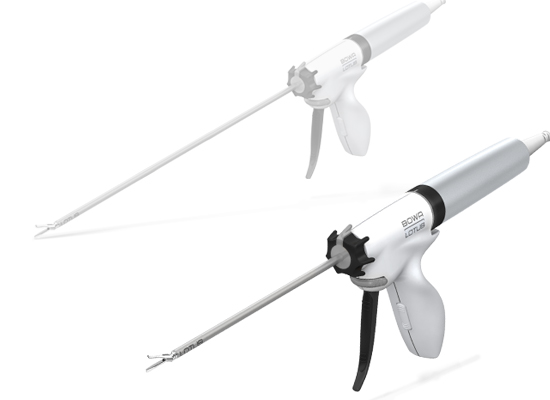
BOWA MEDICAL LOTUS open-surgery resector with unique torsional ultrasound
In conventional ultrasonic knives, energy is directed lengthways through the tip of the instrument. This leads to high energy dissipation losses at the instrument tip and can harbour the risk of unintentional distal penetration of the tissue.The patented torsional ultrasound technology makes the LOTUS liver resector particularly efficient and safe. The energy output of the LOTUS system is transverse to the instrument. This ensures a controlled energy orientation at the instrument tip. The energy dissipation and unwanted distal penetration of the tissue are significantly reduced.

Tips on using the ultrasound transducer for liver surgery
In addition to the LOTUS transducer, to use the LOTUS open-surgery liver resector, you will also need the single-use open-surgery 200 (LR4-200SD) straight hand piece. Transducers have an actual activation time of 250 minutes.Scope of delivery
Incl. instructions for useSize / Dimensions
176 mm
Cable
3 m
Shape
Straight
Total gross weight
493 G
Net unit weight
253 G
Single-use / reusable
Reusable, 250 minutes activation time
Sterile / non-sterile
Non-sterile
Cleaning / Disinfecting
Autoclave
Preparation
Max. 50 cycles
Permissible combinations
LG4, LR4-200SD
Packaging unit
PCS (1 PCS)
Scope of delivery
Incl. instructions for use
CE conformity marking
YES
Notified body
SGS Belgium NV (1639)
EU medical device classification
IIb
Manufacturer
SRA Developments Ltd
Do you still have any questions regarding the product?
We will be happy to help you!
