BOWA MEDICAL neutral electrodes for optimal contact with the patient
Disposable neutral electrodes from BOWA MEDICAL ensure optimal contact between the electrosurgical unit and the patient. The modern hydrogel and an all-round electrode distribution offer a high level of patient safety. The combination of the new anatomically optimised design and the liquid-repellent exterior allows for ideal body adaptation and protection.
Single-use return plates with connector cable
The neutral electrodes come with useful constructive features, such as strain relief, safety edges and an optimised cable. The pre-connected cable has an extremely flat connection. This minimises the risk of pressure points at connection points on the patient's body.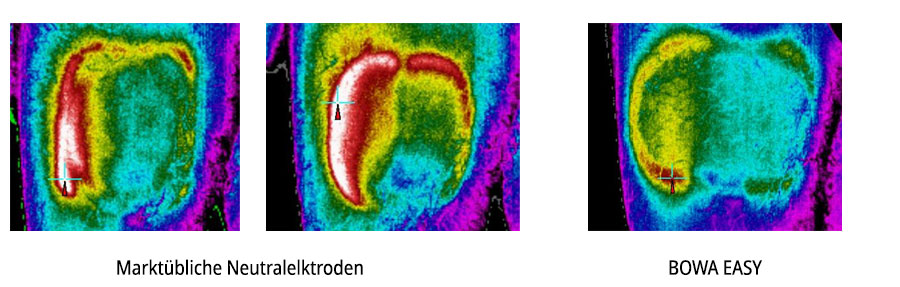
Maximum patient safety thanks to the low-temperature solution
BOWA MEDICAL's neutral electrodes offer ideal thermal properties. The surface area and special design of the neutral electrode reduces the current density and thus the local heating to a minimum.The thin hydrogel layer ensures faster heat dissipation and significantly lower heating compared to the current market standard. The products carry a CE mark as per the 93 / 42 COC guideline and are compliant with DIN EN ISO 10993 and IEC 60601-2-2.
Innovative, safe and economical – product details of the neutral electrodes
Advantages of the BOWA MEDICAL neutral electrodes:- Innovative hydrogel: Extra thin hydrogel layer for low heat storage, optimal skin contact and absorption of moisture
- Body adaptation: Elastic foam cover for an ideal adaptation to the body shape
- Liquid-repellent: Additional protection through liquid-repellent exterior
- Safeguarding: Bonded safety edges for optimal fixation and isolation
- All-round safe: Safety ring for optimal current symmetry and ideal support for modern contact-quality-monitoring systems
Size / Dimensions
90 cm²
Cable
Without cable
Total gross weight
900 G
Net unit weight
6 G
Monopolar / bipolar / passive
Passive
Single-use / reusable
Single-use
Sterile / non-sterile
Non-sterile
Preparation
Not permissible
Permissible combinations
101-150, 294-050, 380-050, 386-050, Permitted generators see compatibility list
Packaging unit
PAC (100 PCS)
CE conformity marking
YES
Notified body
TÜV SÜD Product Service GmbH (0123)
EU medical device classification
IIb
Manufacturer
BOWA-electronic GmbH & Co. KG
Note
split, universal > 5 kg
Do you still have any questions regarding the product?
We will be happy to help you!
